beetrootCANCER and PSFU & RMS
beetrootCANCER has grown to become a fully-fledged digital cancer information system with the added benefit of sophisticated monitoring pathways for a growing range of specialties (other than tumour sites). This growth has been achieved through collaborative development with clinicians and managers working at three very different hospitals.

Problem…
As patients receive ever better care for cancer diagnoses their life expectancy improves. They can return to living normal lives. However there is always a risk that their cancer may return. Should it do so they need the earliest possible intervention. While they’re away from the hospital environment living independent lives its easy for those earlier signs to be missed.
Additionally there is an increased risk that newly referred patients whose investigations have been deferred due to capacity issues may be ‘lost’ to the system as it gets back to normal. Delays can prove life-threatening as shown in this article.
Solution…
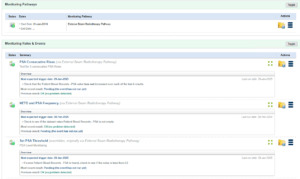
Utilise digital technology to automate a Remote Monitoring Surveillance (RMS) that conforms to the requirements of Personalised Stratified Follow Up (PSFU), as recommended by the NHS.
Collect a range of data from patients, blood and other biomarker tests, endoscopy, histology, radiology reports, treatment details, quality of life questionnaires like HNAs, (perhaps using beetroot@). Set up patient-specific monitoring pathways that enable automatic identification of patients of concern. Monitoring pathways are similar to the monitoring profiles used in beetrootDMARD, the monitoring rules for which which are based on diagnosis and medication. In beetrootCANCER the templates’ rules are based on tumour site and treatment regime, for example urology and prostate cancer.
Also use beetroot@ to:
- collect remote data from patients such as quality of life questionnaires and some vital signs and use those data to identify early onset of problems
- provide regular ‘nudge’ education and advice
- enable two-way communication between patients and their clinicians
Who uses beetrootCANCER?
beetrootCANCER is used by a growing number of tumour sites across three hospitals. Its flexible design lends itself to meeting often very different needs of individual tumour sites. This list is ever growing – Cancer of Unknown origin (CUP) and Sarcoma being added…
Why use beetrootCANCER?





